How Do Heterogeneous Catalysts Work
Homogeneous catalysts are those which exist in the same phase gas or liquid as the reactants while heterogeneous catalysts are not in the same phase as the reactants. Catalysts in the same phase are called homogeneous catalysts while those in different phases are called heterogeneous catalysts.
how do heterogeneous catalysts work is important information accompanied by photo and HD pictures sourced from all websites in the world. Download this image for free in High-Definition resolution the choice "download button" below. If you do not find the exact resolution you are looking for, then go for a native or higher resolution.
 Chapter 14 Chemical Kinetics Part 6 Of 17 Youtube
Chapter 14 Chemical Kinetics Part 6 Of 17 Youtube
So how do catalysts work.
How do heterogeneous catalysts work. Phase distinguishes between not only solid liquid and gas components but also immiscible mixtures eg. Catalysts can be divided into. A reaction catalysed by a heterogeneous catalyst can be represented by a flow chart and animated diagram.
Typically heterogeneous catalysis involves the use of solid catalysts placed in a liquid reaction mixture. Oil and water or anywhere an interface is present. The principles of catalysis.
The modification of homogeneous catalysts is easy. Johnson matthey catalysts. Heterogeneous catalysis is the type of catalysis where the phase of the catalyst differs from the phase of the reactants or productscontrasts with homogeneous catalysis where the reactants products and catalyst exist in the same phase.
Well its quite simple really. How catalysts work catalysts permit an alternate mechanism for the reactants to become products with a lower activation energy and different transition state. A catalyst may allow a reaction to proceed at a lower temperature or increase the reaction rate or selectivity.
Reactant catalyst. The modification of heterogeneous catalysts is difficult. Heterogeneous catalysts work better in high temperature conditions around 250 to 500 c.
Catalysts are compounds that can increase the reaction rate of a particular reaction to give an optimum yield in a short. This page looks at the the different types of catalyst heterogeneous and homogeneous with examples of each kind and explanations of how they work. How does a heterogeneous catalyst work.
For example if we have pt metal as a catalyst for the reaction of hydrogen gas and ethene gas then the pt is a heterogeneous catalyst. There are two main groups of catalysts heterogeneous and homogeneous. You will also find a description of one example of autocatalysis a reaction which is catalysed by one of its products.
The intermediate product then reacts with the other reactant to form the final product. Catalysts can be classified into two types. Summary homogeneous vs heterogeneous catalyst.
In this lesson well learn how they are different how each type reacts. When a homogeneous catalyst is present one of the reactants substrate reacts with the catalyst forming an intermediate product.
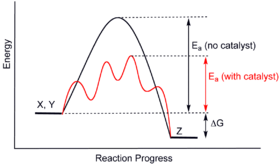 Catalysis Wikipedia
Catalysis Wikipedia
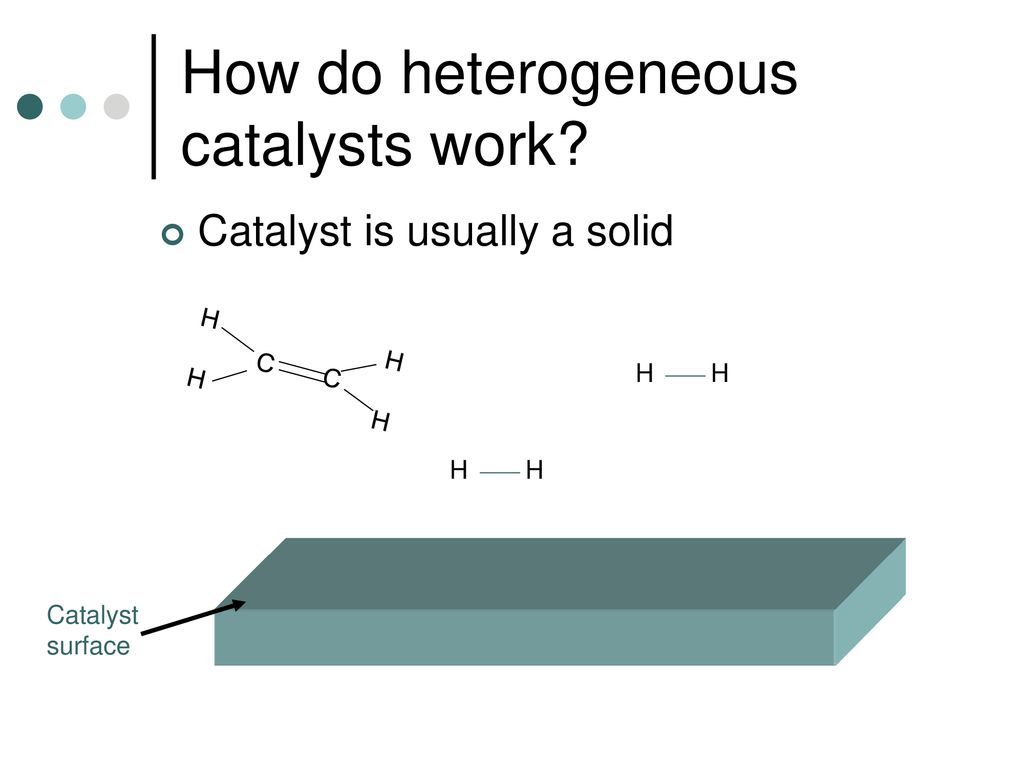 Ci 10 4 What Is A Catalyst Ppt Download
Ci 10 4 What Is A Catalyst Ppt Download
 Catalyst Facts Summary Definition Chemistry Revision
Catalyst Facts Summary Definition Chemistry Revision
 Catalysts Free Full Text Heterogeneous Catalysis On Metal
Catalysts Free Full Text Heterogeneous Catalysis On Metal
 Bridging Homogeneous And Heterogeneous Catalysis By Heterogeneous
Bridging Homogeneous And Heterogeneous Catalysis By Heterogeneous
 Solid Phase Catalysis In Continuous Flow Syrris Chemistry Blog
Solid Phase Catalysis In Continuous Flow Syrris Chemistry Blog
 Oxalate Degradation A And Toc Reduction B Under Different
Oxalate Degradation A And Toc Reduction B Under Different
 Catalysis Boundless Chemistry
Catalysis Boundless Chemistry
 Inorganic Solids And Heterogeneous Catalysis 2012 2013 M Sc
Inorganic Solids And Heterogeneous Catalysis 2012 2013 M Sc

0 Response to "How Do Heterogeneous Catalysts Work"
Post a Comment